Definitions:
1) Heat is the amount of kinetic energy inside a certain object.
2) Temperature is the degree or amount of heat present in an object.
3) Standard Enthalpy Change of Reaction is the enthalpy change that occurs in a system one mole of matter is transformed by a chemical reaction under standard reactions.
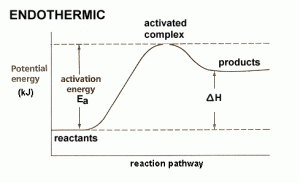
4) Endothermic Reaction is the amount of heat to require to break the reactant bonds.
5) Exothermic Reaction is the amount of heat released when new bonds are formed.
6) The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state.
7) The standard enthalpy of combustion is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at constant pressure.
8) Bond energy is the measure of bond strength in a chemical bond.
9) Standard enthalpy of atomization (or vaporization) is the energy required to transform a given quantity of a substance into a gas at a given pressure (often atmospheric pressure).
10) Electron Affinity is the enthalpy change that occurs when one electron is gained by each atom in a mole of gaseous atoms of the element to give one mole of ions, each with a single negative charge, at standard temperature and pressure.
11) Ionization energy is the energy required to remove an electron from a gaseous atom or ion.
12) Lattice Enthalpy is the enthalpy change involved in formation of the ionic compound from gaseous ions.
13) Entropy is a thermodynamic quantity representing the unavailability of a system’s thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system.
14) A spontaneous reaction takes place when the reaction releases heat to increase stability
15) Gibbs Free energy is defined as a measure of the total change of entropy that results into a exothermic reaction.
16) DG = DH – TDS is the equation for Gibbs free energy which states that the total change of entropy (Gibbs free energy) is equal to the difference between the enthalpy change and the product of the temperature and entropy change.
Sources:
Wikipedia
http://www.ausetute.com.au/heatcomb.html
http://www.chemguide.co.uk/physical/energetics/definitions.html
http://www.science.uwaterloo.ca/~cchieh/cact/c120/heatreac.html